Advancing Quality Care for NSCLC with Actionable Alterations
Welcome to the CME resource center for the NSCLC ONCOdriver Therapy Tool. 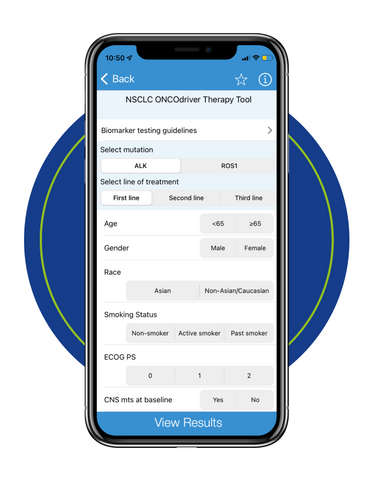
The NSCLC ONCOdriver Therapy Tool provides expert recommendations for the management of patients with non-small cell lung cancer (NSCLC) based on molecular biomarker testing and the presence of actionable oncodriver mutations (eg, ALK, ROS1). To support healthcare professionals in the effective use of the tool, the clinical resources listed below offer concise summaries of key aspects of biomarker-directed care.
After using the NSCLC ONCOdriver Therapy Tool and viewing the clinical resources, you will have the opportunity to tell us whether these resources confirmed or changed your NSCLC management choices. Please contact us if you have any questions: cme@cme-cpd.org.
NSCLC ONCOdriver Therapy Tool Methodology
The NSCLC OncoDriver Therapy Tool was developed in collaboration with ONCOassist as part of a CME initiative supported by independent medical educational grants from Pfizer and Takeda Pharmaceuticals U.S.A., Inc. The tool integrates guideline recommendations on biomarker testing and NSCLC treatment from the American Society of Clinical Oncology (ASCO), European Society of Medical Oncology (ESMO), and National Comprehensive Cancer Network (NCCN).
All ONCOassist tools are designed by oncologists for oncologists. To develop the NSCLC ONCOdriver Therapy Tool, a team of ONCOassist medical oncologists reviewed the ASCO, ESMO, and NCCN guidelines for NSCLC biomarker testing and management. With practical guidance, the NSCLC ONCOdriver Therapy Tool helps oncologists, nurses, and other members of the cancer team make evidence-based decisions at the point of care.